Glyphosate herbicide disrupts the development of the uterus of female rats when they are exposed for 7 days after birth, a new study by Argentine researchers shows.
The glyphosate herbicide caused cell proliferation and structural changes in the rats’ uterus. This was in spite of the fact that no signs of chronic or acute toxicity or differences in weight gain were seen in treated pups.
Glyphosate herbicide also disrupted the expression of proteins involved in uterine development.
The authors conclude that exposure to glyphosate herbicide may affect female fertility and/or promote the development of uterine cancer.
They also state that their study is the first to show endocrine-disrupting effects of a glyphosate-based herbicide on the uterus of newborn and prepubertal rats, supporting the possibility that glyphosate-based herbicides might be endocrine disruptors.
Neonatal exposure to a glyphosate based herbicide alters the development of the rat uterus
Full Paper: http://www.sciencedirect.com/science/article/pii/S0300483X16300932
Authors: Marlise Guerrero Schimpf, María M. Milesi, Paola I. Ingaramo, Enrique H. Luque, Jorgelina Varayoud
Abstract:
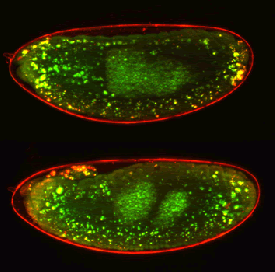
Glyphosate-based herbicides (GBHs) are extensively used to control weeds on both cropland and non-cropland areas. No reports are available regarding the effects of GBHs exposure on uterine development. We evaluated if neonatal exposure to a GBH affects uterine morphology, proliferation and expression of proteins that regulate uterine organogenetic differentiation in rats. Female Wistar pups received saline solution (control, C) or a commercial formulation of glyphosate (GBH, 2 mg/kg) by sc injection every 48 h from postnatal day (PND) 1 to PND7. Rats were sacrificed on PND8 (neonatal period) and PND21 (prepubertal period) to evaluate acute and short-term effects, respectively. The uterine morphology was evaluated in hematoxylin and eosin stained sections. The epithelial and stromal immunophenotypes were established by assessing the expression of luminal epithelial protein (cytokeratin 8; CK8), basal epithelial proteins (p63 and pan cytokeratin CK1, 5, 10 and 14); and vimentin by immunohistochemistry (IHC). To investigate changes on proteins that regulate uterine organogenetic differentiation we evaluated the expression of estrogen receptor alpha (ERα), progesterone receptor (PR), Hoxa10 and Wnt7a by IHC. The GBH-exposed uteri showed morphological changes, characterized by an increase in the incidence of luminal epithelial hyperplasia (LEH) and an increase in the stromal and myometrial thickness. The epithelial cells showed a positive immunostaining for CK8, while the stromal cells for vimentin. GBH treatment increased cell proliferation in the luminal and stromal compartment on PND8, without changes on PND21. GBH treatment also altered the expression of proteins involved in uterine organogenetic differentiation. PR and Hoxa10 were deregulated both immediately and two weeks after the exposure. ERα was induced in the stromal compartment on PND8, and was downregulated in the luminal epithelial cells of gyphosate-exposed animals on PND21. GBH treatment also increased the expression of Wnt7a in the stromal and glandular epithelial cells on PND21. Neonatal exposure to GBH disrupts the postnatal uterine development at the neonatal and prepubertal period. All these changes may alter the functional differentiation of the uterus, affecting the female fertility and/or promoting the development of neoplasias.

























































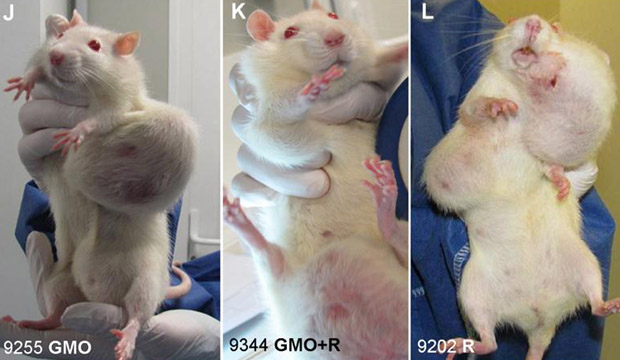








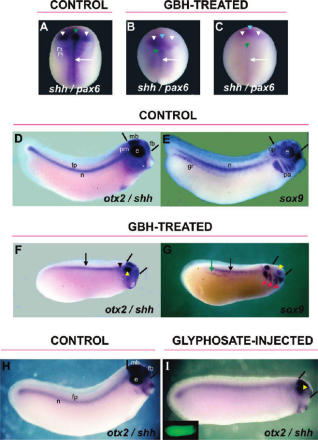





What you guys fail to understand is that this was the plan all along. Businessmen founded the American Medical Association, founded the medical schools and put one of their cronies on the board to guide the curriculum. They knew they couldn’t patent natural remedies and nutrition.
Do you know how much nutrition doctors get? Almost zero! That is because the elite, corporate fascists know that the only thing that can cure disease is nutrition. When you give the body what it needs to heal, it will. Google Max Gerson. He is mysteriously dead now. Just like anyone who tries to inspire change or dream.
The systematic poisoning of the American people is moving along with break-neck speed and they have you so distracted by sports, fake news, social media and false shootings, you don’t care. Not that they are gonna tell you that you are polluted.
Ninety percent of the media in this country is owned by 5-6 corporations. They control almost everything American’s see and hear. They want you scared. They want you distracted, they want you programmed by fake news stories and hanging on every word. Notice how they creating an atmosphere of fear? Fear your gay neighbor, fear your Muslim neighbor, fear the police, fear ebola, fear Zika (google how Zika is really a birth defect that was caused by polluting the Brazilian water with larvicide)and fear anything not like you. They know that distracted, fearful, paranoid citizens are easier to control. Please wake up now! It is all a bad dream. But, it is not. The minute they wheeled that TV in front of us all, the division and distraction began. We are losing our liberties at an alarming rate, yet they tell us that Bruce Jenner made man of the year? What about the TPP? What about the chemicals they are spraying in the sky?
Yes! Chemtrails are real. Doubt me? I have marched with a past-presidential candidate for the Green Party over this issue. Where have all the thunder and lightning gone? What are all those lines in the sky? Clouds don’t criss-cross. Don’t tell me those are contrails. Did planes change since I was young? No! Wake up America.
Polluted organisms malfunction; polluted by chemically-laden foods, Fluoridated water, bad pharmaceuticals, geoengineering. The diseases on the rise are not infectious, they are our bodies malfunctioning. Cancer, Parkinson’s, auto-immune, dementia, autism, IBS; the list goes on and on. The prediction is that 50% of American’s will experience cancer in their lifetime. So…why are we racing for the cure and not asking what is causing all this sickness? Do you realize that Susan G. Komen’s CEOmade almost $600, 000 dollars one year. The money we all raise for cancer research goes straight into Bigpharmas wallet. They are laughing at us all the way to the bank.
I have been a Nurse for twenty years. I have worked in many major cities in almost every department of the hospital. We are getting sicker and sicker evey year and nobody is asking the right questions. Look around! What two industries are thriving? The health-care industry and big banks. Get the picture?
The FDA, CDC and EPA and US government have gone rogue. NOBODY has our backs. Google CDC corruption, Dr Hooker, Dr Thompson vaccine fraud. Google who heads the FDA now and the ties he has to big pharma. Google the revolving door of the FDA.
The American people have been sold out. At this point, any intelligent person with half a brain has to acknowledge the fact that we are a resource. We are worth more sick than well, and they know it.
We better wise up and speak up. We should all pray this insanity ends. Please continue this discussion. Talk to your doctor, your lawyer, your neighbor and your children. Speak up and DO NOT CONSENT to this insanity any longer. It starts with you. You may be only one voice, but we can quickly become many.
AwakeNancyRN